Fahd Ahmed1 Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â BDS, MSc
Haroon Rashid2Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â BDS, MDSc
Sadaf Farookhi3Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â BDS, MSc
Vivek Verma4Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â BDS, MSc
Yuliya Mulyar5Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â BA
Murai Khalifa6Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â BDS, DDS
Zeeshan Sheikh7Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Dip, Dh, BDS, MSc, PhDÂ Â Â Â Â Â Â Â Â
ABSTRACT:
The rate of dental implants osseo-intergration is strongly related the implant material composition and surface roughness. It is believed that implants with roughened surfaces offer better bone anchoring and biomechanical stability. This article presents an overview of the surface modifications that are carried out to improve and promote osseo-integration. There are various methods for enhancing the surface roughness and to improve the osteoconductive coatings. This paper discusses the processes of grit-blasting, plasma spraying, acid-etching, anodization and implant surface coatings with hydroxyapatite. The modifications described here are essentially methods employed to improve the osseo-integrative ability of titanium implants. Although the precise role surface topography plays in osseo-integration remains unclear, it is required in future that surfaces with controlled and standardized topography are developed and the process of osseo-integration of implants is enhanced to achieve longterm clinical success.
KEY WORDS: 1.Ossointegration;Â 2. Implant surfaces;Â 3. Implant surface modifications; 4. Hydroxyapatites.
HOW TO CITE: Ahmed F, Rashid H, Farookhi S, Verma V, Mulyar Y, Khalifa M, Sheikh Z. Surface modifications of endosseous dental implants by incorporation of roughness and hydroxyapatite coatings. J Pak Dent Assoc 2015; 24(4):162-171.
Received: October 11 2015, Accepted: November 17 2015
INTRODUCTION TO SURFACE TREATMENTS OF IMPLANTS
In the past few decades, there has been a great increase in the number of dental implants being placed. It has been well established already that the success of dental implants is dependent on early as well as late osseointegration.1Â The surface topography and geometry of an implant are important for their long and short term clinical success. For implant fabrication, titanium is the material of choice2,3Â as it has a low density giving it a high-strength-to-weight ratio. For that reason, it can be effectively alloyed with other metals like vanadium (4%) and aluminum (6%) particularly.4Â The strength of the alloy is increased by incorporating aluminum and vanadium, while acting as a scavenger, prevents corrosion.5
Surface modifications like passivation, anodization, ion-implantation and texturing are essential methods for making dental implants perform better in terms of physical and biological standpoint after implantation.6,7 It is believed that surface treatments improve the bioactivity and the osseointegrative properties of Titanium.8-10 Surface texturing can increase implant surface area by up to six times, thereby improving osseointegration10. Implants can also be coated with a variety of materials that can aid implantbone bonding.11 Calcium phosphate-coated implants have been described in the literature which demonstrate early direct bone attachment to the hydroxyapatite surface and this occursas quickly as one month post-operatively.12 The aim of the current review is to discuss various implant surfaces and methods that could accelerate the osseointegration process of dental implants.
METHODS TO IMPROVE SURFACE ROUGHNESS OF IMPLANTS
Numerous reports in the literature have suggested that implant surface characterization affects the rate by which bio-mechanical fixation and osseointegration occurs.13,14 There are three different levels of surface roughness which depend on macro-, micro- and nano-sized topographies. Reports have suggested that the early fixation rates and long-term mechanical stabilization of the prosthesis may be enhanced by a high degree of surface roughness as compared to decreased roughness or smooth surfaces.8,15-17 The surface roughness of dental implants can actually be created by subjecting the surfaces to various treatment methods. The methods of incorporating surface roughness are described in subsequent sections.
Figure 1:Â Various dental implant surface modification techniques.
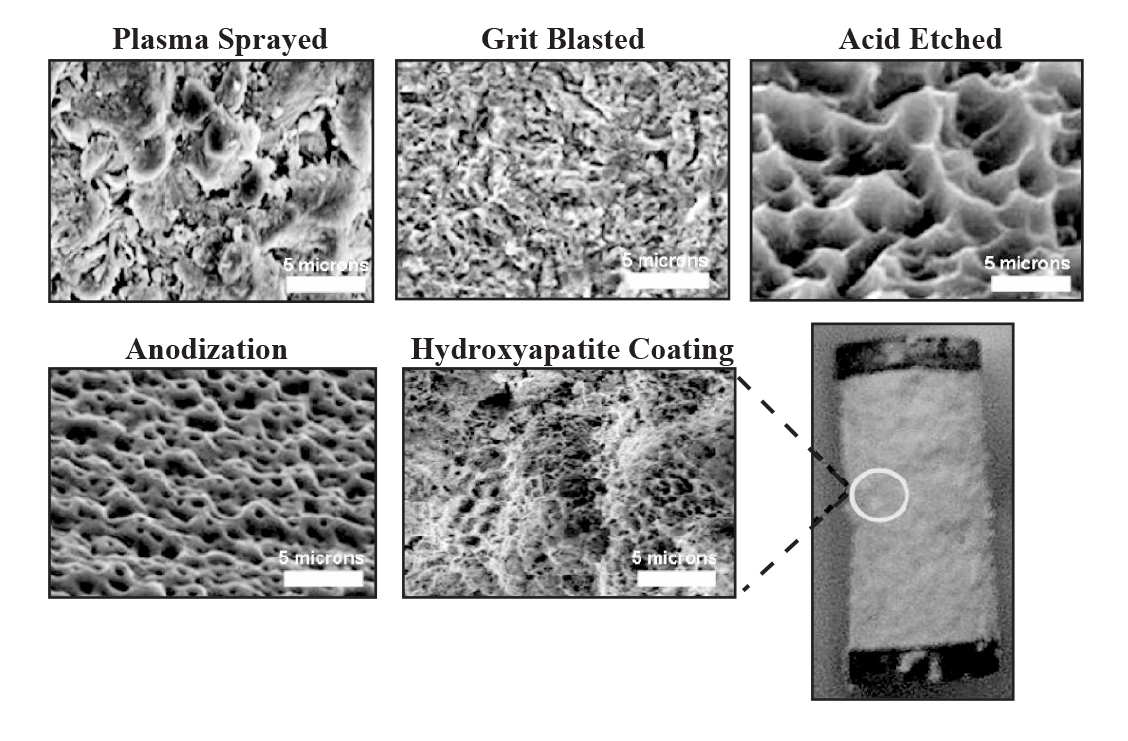
PLASMA SPRAYING
This is a method in which powdered form of titanium is injected into a plasma torch at very high temperatures. These powdered particles that get condensed upon the surface of the substrate ultimately fuse together to form a thin film over the implant surface.8 Usually, the plasma sprayed film is of approximately ~30 µm thickness. The film provides roughness of about ~3-7 µm which also increases the surface area.8 The 3D topography results in an increase of the tensile strength at the implant-bone interface.18 Preclinical studies on mini pigs have shown faster bone formation around the roughened implant surfaces as compared to the implant surfaces that are smooth with an average roughness of about 0.2 µm.19 Prospective case series studying treatment outcomes of titanium plasma sprayed implants reported that 92% of implants showed less than 1mm of peri-implant bone loss over a period of 20 years.8
GRIT BLASTING
This is the process in which bombarding of the titanium surfaces is done using hard ceramic particles which are forced through nozzle by using compressed air at very high velocities.20 This results in surfaces with varying degrees of roughness and the variance is dependent upon the sizes of ceramic particles used on the implant surfaces, the air pressure and hardness values of the actual implant surface itself .21 Commonest materials used as grit include alumina, calcium phosphate and titanium oxide. They are biocompatible and are believed to cause no interference with the osseointegration of titanium implants post operatively.22,23
Titanium implant surfaces blasted with 25 µm titanium oxide particles result in a moderately rough surface of about 1-2 µm.18,24 Studies have shown that those implants which have been grit blasted using titanium oxide show greater contact with the bone during osseointegration24 and this has been further proven by comparing their levels of osseointegration with machined surfaces.8,25 Clinical trials have also shown that these implants have a success rate of 97.6% at 5 years and 91.1% at 6 years after implantation.24,26 A study model demonstrated by Wennerbergand Albrektssonet al. showed implants which have been grit blasted using titanium oxide or alumina have similar bone-implant contact as compared to smooth titanium implants, but with an increased biomechanical fixation.18 Also, the ability of an implant to withstand torque force increases with an increase in its surface roughness.8 Calcium phosphates i.e. hydroxyapatite and β-tricalcium phosphate and their mixtures have also been used to grit blast titanium implant surfaces.23 Calcium phosphates are osteoconductive and biocompatible and have shown to be resorbable with time.27-31 Calcium phosphate grit blasted implant surfaces have also been shown to achieve greater implant to bone contact as compared to those surfaces that have only been machined.32
ACID ETCHING
Hydrochloric acid, hydrosulphuric acid, nitric acid and hydrofluoric acid are strong acids which have been used for roughening of titanium implant surfaces.33,34 Micropits are with sizes ranging from 0.5 to 2 µm in diameter are produced over the surfaces and this results in an increase in the surface area.8 This also causes superior bone adhesion and thus, increases the rate of osseointegration.35 The process of acid Etching is done by immersing the implant into a mixture of concentrated acids for a period of several minutes and then subjecting the implant surface to the process of heating above 100 °C36 The process carried out at high temperatures produces micro porosities which are homogenous in nature that helps in better wettability, superior contact with the bone, good fibrin attachment and enhanced osseointegration subsequently.36 Other advantages include less contamination since no surface particles are produced, and better osteoblastic retention. The osseo-conductive process has been shown to get enhanced by the use of dual acid etching process that promotes attachment of fibrin and osteogenic cells resulting in formation of the bone directly over the implant surface.37 However; the use of acids also negatively affect the mechanical properties and thus, creating micro-cracks over the surfaces causing the reduction of fatigue resistance of the implant.8 It has been shown that commercially pure titanium with concentration of hydrofluoric acid 48% at 90 °C for 15 min to 8 hours results in high values of surface roughness and increases sub-surface hydrogen.38 Sulphuric acid has been shown to produce more surface roughness than other acids like hydrochloric acid and nitric acid.38 A study conducted in dogs found that implants having dual acid etched surfaces had higher torque to interface failure compared to implants with as-machined surfaces.39
ANODIZATION
Micro or nano-porous surfaces can be created by the potentiostatic or galvanostatic anodization of titanium in acids such as hydrosulphuric acid, hydrophosphoric acid, nitric acid and hydrofluoric acid.40 The process of anodization also causes an increase in the oxide layer thickness to more than 1000 nm over titanium surface .41,42 Because the acids used in the electrolyte solution are strong, this oxide layer is dissolved and is also thickened in other areas. The dissolution is also responsible for generating micro or nano-pores on the titanium surface43, creating a moderately rough surface that facilitates osteoblast cell adhesion.44
Anodized surfaces display better bone reinforcement and the osseointegration taking place on such surfaces can be explained either because of the mechanical interlocking in the pores by bone growth or due to biochemical bonding.42 Certain chemicals like calcium, magnesium and phosphorous have been incorporated in surface oxide layer.43 Another process called anodic spark deposition has been investigated for its role in enhancement of titanium biocompatibility. A comparison has been done between Anodized alkali treated (AAT) titanium with chemically etched surfaces (bio-rough) and machined implant surfaces.43 The surfaces which were chemically etched showed improved surface morphologies and caused no chemical modifications over the titanium implants. However; those surfaces which were subjected to AAT, showed bioactive micro- and nano-porous Ca and PO4 enriched titanium oxide layer having a crystalline anatase structure.43 This structure has shown to encourage hydroxyapatite layer nucleation and enhances the deposition of Ca and PO4. It has been shown in experiments that the AAT surfaces encouraged proliferation and adhesion of cells over a period of 2 weeks and may be used in achieving stable and faster osseointegration of endosseous dental implants.46
CERAMIC COATINGS ON DENTAL IMPLANTS
Materials like bioceramics (calcium phosphates) can be used to modify surface of a dental implant.47 Bioactive ceramic implants are inorganic materials and depending upon on the physico-chemical nature of coatings, this can accelerate the formation rate of bone implant interface and also increases its stability and strength. The use of ceramics for coating metallic implants that produce an ionic ceramic surface has been documented.48 The materials can be plasma sprayed or may be coated on to an implant surface. However it is worth mentioning that ceramics are brittle in nature, having low tensile and compressive strength.10
HYDROXYAPATITE (HA)
Hydroxyapatite (HA) is a compound of calcium phosphate with a composition of Ca10 (PO4)6 (OH)2 and has a crystallographic structure. It has a space group of p63/m and has a hexagonal atomic arrangement. This space group shows a six-fold c-axis perpendicular to the three equivalent a-axes at angles of 120 degree to one another. Every building cell or unit contains Ca, PO4 and OH groups with are densely packed with each other.10 The appearance of Ca ions is in a form of a triangle while the OH groups are at the corner positions and the phosphates tetrahedral show a helical arrangement. Fluoride and chloride ions can be used to substitute the OH group which may cause a change in properties like the morphology, solubility and lattice parameters without the change in hexagonal parameters. For example, substituting the OH group with fluoride ions causes the contraction of the cell which in turn increases crystallinity and the size of the crystal.49 This substitution results in less soluble and more stable structure.50
METHODS OF SYNTHESIZING HYDROXYAPATITE
There are many ways by which hydroxyapatite can be synthesized. Following are the common methods [52]:
- Wet Methods which include precipitation method, hydrothermal techniques and hydrolysis of calcium phosphate.
- Solid state reactions.
- Ultrasonic irradiation
- Sol-gel method
- Microwave irradiation
- Chemical precipitation
- Micro-emulsion.
Depending on the technique used, differing morphological, stoichiometrical and crystallized products be obtained and authors feel that it is beyond the scope of this article to review all preparation methods.
HYDROXYAPATITE COATINGS
Since hydroxyapatite has a porous structure and is similar to the inorganic structure of teeth and bones, is has been widely researched upon for its use as a coating over metallic implants.21 However, its bulk is quite brittle if compared with ceramics zirconia oxide and aluminium.44 HA coatings help to provide early stabilization of the dental implant in the surrounding bone and thus significantly reducing the healing time so that the prosthetic implant component can be placed earlier. It increases the functional life of the prosthesis over the implant and also produces optimum tissue response51 so that when the breakdown of the coatings occur, there is no adverse reaction in tissues surrounding the implant. The dissolution rate of HA in both alkaline and neutral aqueous solutions is quite low.31,51 It has been speculated that even thought there is disintegration of the released PO4 and Ca ions around the implant area, negative effects on bone formation are minimum. However; HA could cause disruption of the passive oxide layer present on the titanium implant surface. This disruption in turn causes the release of metallic ions which is undesirable.52
It is generally believed that larger the crystallinity of the HA coating, the greater would be the resistance to dissolution in vivo.53 On the other hand, HA surface coatings which are amorphous in nature would demonstrate a substantially higher rates of dissolution.54 50% crytallinity of HA is thought to be ideal for dental implant coatings.55 Literature has shown that HA coated implants demonstrate better implant to bone integration. The values have been compared and for HA coated implants, these range between 17.1% for 7 days to 75.9% for 3 months as compared to values for metallic titanium implants which range from 1.2% in 7 days to 45.7%in 3 months19,56 Although HA coated implants show better torque resistance is short term, it has also been shown that after a period of 6 months, no significant difference in the integration between ceramic coated metallic implants than non-coated metallic implants is seen.57 Also, it is worth mentioning that the coating of HA helps in creating an additional interface between the bone and implant surface and this could be a potential reason for the failure.58 The use of nano-crystalline HA powders for coating has been described in the literature having a number of beneficial properties i.e. improved sintering ability, increased surface area and better densification which helps to reduce the sintering temperature.59,60
METHODS TO COAT IMPLANT SUBSTRATE WITH HYDROXYAPATITE
There are a variety of methods that can be employed to coat implants with HA. However, the minimal requirements of HA being used as dental implant coating are that the crystallinity should be 62 %, phase purity around 95% , density should be 2.98 g/cm3, Ca/P ratio should be 1.67-1.76 , shear strength should be more than 22MPa and tensile strength more than 50.8 MPa.61 Methods used to coat the titanium dental implants with calcium hydroxyapatite with their merits and demerits are presented in Table 1 and discussed in the following sections.
PLASMA SPRAYING
Plasma spraying has been used to create rough surfaces and can also be used to coat dental implants with HA. This technique has similarities to the procedure in which surface porosities are created. It involves the injection of the ceramic HA into the plasma torch at very high temperatures which is then projected on to the surfaces
 of the implant.63 The particles will then condense and fuse resulting in formation of a thin layer on the titanium surface. There is a variation in thickness of this layer and it ranges from a few microns to a few millimetres.8 Plasma spraying has an advantage that it is rapidly deposited and has low cost58, leading to faster osteointegration and decreased healing time.21 As plasma spraying leads to mechanical bonding of Ca/P with the substrate, roughening of the substrate gives good mechanical interlocking64, which allows for an extended functional life of the implant.8 Disadvantages related to plasma spraying include weak adhesion9,21 the formation of the residual stresses which may be created at the interface between the substrate and the coating. Plasma spraying may not be considered a technique for smaller dental implants with complex designs65as the coating may disintegrate if the implant is insertion into bone with increased density.8
of the implant.63 The particles will then condense and fuse resulting in formation of a thin layer on the titanium surface. There is a variation in thickness of this layer and it ranges from a few microns to a few millimetres.8 Plasma spraying has an advantage that it is rapidly deposited and has low cost58, leading to faster osteointegration and decreased healing time.21 As plasma spraying leads to mechanical bonding of Ca/P with the substrate, roughening of the substrate gives good mechanical interlocking64, which allows for an extended functional life of the implant.8 Disadvantages related to plasma spraying include weak adhesion9,21 the formation of the residual stresses which may be created at the interface between the substrate and the coating. Plasma spraying may not be considered a technique for smaller dental implants with complex designs65as the coating may disintegrate if the implant is insertion into bone with increased density.8
BIOMIMETIC DEPOSITION
Biomimetic deposition method is done under lower temperaturesand the risk of the coating flaking off is minimized. It also allows bone like HA crystals with greater bioactivity to be formed.66 Firstly, oxidization of the substrate is carried out in a furnace at 800°C for a period of 1 hour. The procedure is started at lower temperatures and is increased at a rate of 5 °C per min until a temperature of 800 °C is achieved. This is held constant for 1 hour and then allowed to cool down slowly. These plates are cleaned using an alkaline medium in an ultrasonic bath and are the substrates are then inserted in plastic tubes containing phosphate buffered saline (PBS) solution, for 7 days in an incubator at a temperature of 37 °C. This procedure will cause the HA particle deposition. It has been shown that those implants having layers of HA placed by the process of biomimetic deposition show better bone bonding and thus, enhanced osseointegration.66-68
SPUTTERING TECHNIQUE
Sputtered coatings have been gaining popularity recently because of the problems associated with plasma spraying .63,69 The sputtering of calcium phosphates and HA leads to better bone strength and superior rates of initial osseointegration.63 This process results in the formation of a coating of uniform thickness on flat substrates and this forms a dense coating of 0.5-3 µm thick. The ratio of Ca/P at the surface material is between the range of 1.6-2.6 and this is because of the fact that phosphorous ions are weakly bound onto the surfaces.70 In vivo studies have proven that calcium phosphate coatings which are sputtered showed superior implant fixation and healing response as compared to those surfaces which are grit blasted.63,71 Another study carried out on rat bone marrow stromal cells showed better osteoconductivity and biodegradability on titanium plates with sputtered HA coatings, as compared to surface-modified titanium.22
ELECTROPHORETIC TECHNIQUE (EPD)
The technique has been conventionally used to deposit ceramics, glass, polymers and can also be used to deposit composites materials like chitosan and HA.72 The cell for the process of electrophoretic deposition includes a cathodic substrate which is centred between two parallel platinum counter electrodes. The voltage for electrophoretic deposition is usually 10 to 30 V73 and the average length of the formed HA is 200 nm which is essentially needle shaped.73 The main advantage of this process is that it allows the deposition of coatings that contain HA-chitosan composites andsilica-chitosan.74 This process may be carried out at room temperature that thus, may allow codeposition of various materials.75 Studies have found that EPD-HA coatings have higher corrosion resistance as compared to uncoated titanium and also greater adhesion strength than plasma sprayed HA coatings.76
DEPOSITION BY ELECTROCHEMICAL PROCESS
Depositing of HA using the electrochemical ways has gained much popularity as it can be used to coat those metal substrates which are highly irregular. The process is done quickly and at low temperatures. The chemical composition and the thickness of the coatings can be controlled provided that there are adequate conditions present during the process.77 The specimens are first wetground using an appropriate abrasive paper, polished and rinsed using acetone and then lastly washed with distilled water. The deposition is then carried out using a potentiostat/galvanostatic cathodic polarization. The substrate during the process is used as cathode and platinum plate is used as the counter electrode.66 The polarization during the process is conducted from an open circuit voltage (-3.0V) at the rate of -0.6 V per hour. The specimen is rinsed in distilled water so that the residual electrolytes are removed and then allowed to dry for 24 hours. This technique results in greater amount of hydroxyl from reduction of water and forms HA coatings which are more crystalline in nature. Thecoatings formed by the process describe above are resistant to high scratch load of about 20N.77,78
CONCLUSION
This paper has discussed surface modification of dental implants by incorporation of roughness and coatings. Clinical and preclinical investigations have demonstrated that the bone response to a dental implant is greatly influenced by the surface topography. However, increased surface roughness of titanium implants could have contributed towards peri-implant disease as well as an increase in ionic leakage. The various techniques described above may lead to different in vivo responses after implantation and could affect clinical success rates. It is not easy to do a comparison particularly because of variation in the techniques used for implant surface characterization. Further in-depth research, especially long-term studies are required before anything definitive can be determined.
Author Contribution:
FA performed original design of the manuscript and writing, HR did peer review of the manuscript, correction of typographical errors and critical analysis, YM, MK, VV and SF critically analysed the manuscript and gave final approval, ZS drafted images and tables and reviewed the manuscript. Disclosure: None disclosed
REFERENCES
- Albrektsson T, Zarb G, Worthington P, Eriksson A. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int j oral maxillofac implants. 1986;1:11-25.
- Watari F, Yokoyama A, Saso F, Uo M, Kawasaki T. Fabrication and properties of functionally graded dental implant. Composites Part B: Engineering. 1997;28:5-11.
- Brunette DM. Titanium in medicine: material science, surface science, engineering, biological responses, and medical applications: Springer Science & Business Media; 2001.
- Elias C, Lima J, Valiev R, Meyers M. Biomedical applications of titanium and its alloys. Jom. 2008;60(3):469.
- Saini M, Singh Y, Arora P, Arora V, Jain K. Implant biomaterials: A comprehensive review. World Journal of Clinical Cases: WJCC. 2015;3:52.
- Tamir S. Method for manufacturing metal with ceramic coating. Google Patents; 2007.
- Alla RK, Ginjupalli K, Upadhya N, Shammas M, Ravi RK, Sekhar R. Surface roughness of implants: a review. Trends Biomat Artif Organs. 2011;25:112-8.
- Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent mater. 2007;23:844-54.
- Elias CN, Oshida Y, Lima JHC, Muller CA. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J mechbehav biomed mater. 2008;1:23442.
- Anusavice KJ, Shen C, Rawls HR. Phillips’ science of dental materials: Elsevier Health Sciences; 2012.
- Puleo D, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311-21.
- Pak H-S, Yeo I-S, Yang J-H. A histomorphometric study of dental implants with different surface characteristics. J adv prosthodont. 2010;2:142-7.
- Cochran D, Schenk R, Lussi A, Higginbottom F, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J biomed mater res. 1998;40:1-11.
- Wennerberg A, Hallgren C, Johansson C, Danelli S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin oral implants res. 1998;9:11-9.
- Steigenga JT, Al-Shammari KF, Nociti FH, Misch CE, Wang H-L. Dental implant design and its relationship to long-term implant success. Implant dent. 2003;12:30617.
- Wennerberg A, Albrektsson T, Andersson B, Krol J. A histomorghometric study of screw-shaped and removal torque titanium implants with three different surface topographies. Clin oral implants res. 1995;6:24-30.
- Gotfredson K, Wennerberg A, Johansson C, Skovgaard LT, Hjørting-Hansen E. Anchorage of TiO2-blasted, HAcoated, and machined implants: An experimental study with rabbits. J biomed mater res. 1995;29:1223-31.
- Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin oral implants res. 2009;20:172-84.
- Buser D, Schenk R, Steinemann S, Fiorellini J, Fox C, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J biomed mater res. 1991;25:889902.
- Salou L, Hoornaert A, Louarn G, Layrolle P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta biomaterialia. 2015;11:494-502.
- Elias CN, Fernandes DJ, Resende CR, Roestel J. Mechanical properties, surface morphology and stability of a modified commercially pure high strength titanium alloy for dental implants. DentMater. 2015;31(2):e1-e13.
- Barfeie A, Wilson J, Rees J. Implant surface characteristics and their effect on osseointegration. Brit dent j. 2015;218:E9-E.
- Narayanan R, Seshadri S, Kwon T, Kim K. Calcium phosphate-based coatings on titanium and its alloys. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;85:279-99.
- Gotfredsen K, Karlsson U. A prospective 5-year study of fixed partial prostheses supported by implants with machined and TiO2 blasted surface. J Prosthodont. 2001;10:2-7.
- Ivanoff CJ, Widmark G, Hallgren C, Sennerby L, Wennerberg A. Histologic evaluation of the bone integration of TiO2 blasted and turned titanium microimplants in humans. Clin oral implants res. 2001;12:128-34.
- Weng D, Jacobson Z, Tarnow D, Hürzeler MB, Faehn O, Sanavi F, et al. A prospective multicenter clinical trial of 3i machined-surface implants: results after 6 years of follow-up. Int j oral maxillofac implants. 2002;18:41723.
- Sheikh ZA, A. Javaid, MA. Abdallah, MN. Bone Replacement Graft Materials in Dentistry. In: Khurshid Z SZ, editor. Dental Biomaterials (Principle and its Application). 2nd ed: Paramount Publishing Enterprise; 2013.
- Sheikh Z, Sima C, Glogauer M. Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar Bone Augmentation. Materials. 2015;8:295393.
- Sheikh Z, Javaid MA, Hamdan N, Hashmi R. Bone regeneration using bone morphogenetic proteins and various biomaterial carriers. Materials. 2015;8(4):1778816.
- Sheikh Z, Geffers M, Christel T, Barralet JE, Gbureck U. Chelate setting of alkali ion substituted calcium phosphates. Ceramics International. 2015.
- Tamimi F, Sheikh Z, Barralet J. Dicalcium phosphate cements: brushite and monetite. Acta Biomater. 2012;8:47487.
- Novaes Jr AB, Souza S, de Oliveira PT, Souza A. Histomorphometric analysis of the bone-implant contact obtained with 4 different implant surface treatments placed side by side in the dog mandible. Int j oral maxillofac implants. 2001;17:377-83.
- Liu X, Chu PK, Ding C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng. 2004;47:49-121.
- Williams KR, Gupta K, Wasilik M. Etch rates for micromachining processing-Part II. J Microelectromechanical Sys. 2003;12:761-78.
- Massaro C, Rotolo P, De Riccardis F, Milella E, Napoli A, Wieland M, et al. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: chemical composition. Journal of Materials Science: Mater Med. 2002;13:535-48.
- Cho S-A, Park K-T. The removal torque of titanium screw inserted in rabbit tibia treated by dual acid etching. Biomaterials. 2003;24:3611-7.
- Park JY, Davies JE. Red blood cell and platelet interactions with titanium implant surfaces. Clin oral implants res. 2000;11:530-9.
- Ban S, Iwaya Y, Kono H, Sato H. Surface modification of titanium by etching in concentrated sulfuric acid. dent mater. 2006;22:1115-20.
- Yan Guo C, Tin Hong Tang A, Pekka Matinlinna J. Insights into surface treatment methods of titanium dental implants. J Adhesion Sci Tech. 2012;26:189-205.
- Roach P, Shirtcliffe NJ, Newton MI. Progess in superhydrophobic surface development. Soft Matter. 2008;4:224-40.
- Yao C, Perla V, McKenzie JL, Slamovich EB, Webster TJ. Anodized Ti and Ti6Al4V possessing nanometer surface features enhances osteoblast adhesion. J Biomed Nanotech. 2005;1:68-73.
- Sul Y-T, Johansson CB, Petronis S, Krozer A, Jeong Y, Wennerberg A, et al. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown:: the oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomater. 2002;23:491-501.
- Sul Y-T, Johansson C, Wennerberg A, Cho L-R, Chang B-S, Albrektsson T. Optimum surface properties of oxidized implants for reinforcement of osseointegration: surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int j oral maxillofac implants. 2004;20:349-59.
- Chappuis V, Buser R, Brägger U, Bornstein MM, Salvi GE, Buser D. Long-Term Outcomes of Dental Implants with a Titanium Plasma-Sprayed Surface: A 20-Year Prospective Case Series Study in Partially Edentulous Patients. Clin implant dent related res. 2013;15:780-90.
- Bauer S, Schmuki P, von der Mark K, Park J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Progress Mater Sci. 2013;58:261326.
- Toledo VAO. Dental implant surface modifications and osteointegration. 2013.
- LeGeros R, Lin S, Rohanizadeh R, Mijares D, LeGeros J. Biphasic calcium phosphate bioceramics: preparation, properties and applications. Journal of materials science: Mater Med. 2003;14:201-9.
- Paital SR, Dahotre NB. Calcium phosphate coatings for bio-implant applications: materials, performance factors, and methodologies. Mater Sci Eng. 2009;66:1-70.
- Bertoni E, Bigi A, Cojazzi G, Gandolfi M, Panzavolta S, Roveri N. Nanocrystals of magnesium and fluoride substituted hydroxyapatite. J Inorganic Biochem. 1998;72:29-35.
- Hench LL. Bioactive materials: the potential for tissue regeneration. J Biomed Mater Res. 1998;41:511-8.
- Ratner BD. Biomaterials science: an introduction to materials in medicine: Academic press; 2004.
- Lacefield WR. Hydroxylapatite coatings. Adv Series Ceramics. 1993;1:223-38.
- Bhadang KA, Gross KA. Influence of fluorapatite on the properties of thermally sprayed hydroxyapatite coatings. Biomaterials. 2004;25:4935-45.
- Chou L, Marek B, Wagner W. Effects of hydroxylapatite coating crystallinity on biosolubility, cell attachment efficiency and proliferation in vitro. Biomaterials. 1999;20:977-85.
- Gert de Lange D, Tadjoedin E. Fate of the HA coating of loaded implants in the augmented sinus floor: a human case study of retrieved implants. Int J PerioRest Dent. 2002;22;287-96.
- Albrektsson T, Brånemark P-I, Hansson H-A, Lindström J. Osseointegrated titanium implants: requirements for ensuring a long-lasting, direct bone-toimplant anchorage in man. Acta Orthopaedica. 1981;52:155-70.
- Gottlander M, Albrektsson T. Histomorphometric analyses of hydroxyapatite-coated and uncoated titanium implants. The importance of the implant design. Clin oral implants res. 1992;3:71-6.
- Ong JL, Chan DC. Hydroxyapatite and their use as coatings in dental implants: a review. Critical Rev Biomed Eng. 1999;28(5&6):1-41.
- Yousefpour M, Afshar A, Yang X, Li X, Yang B, Wu Y, et al. Nano-crystalline growth of electrochemically deposited apatite coating on pure titanium. J Electroanalytical Chem. 2006;589:96-105.
- Mobasherpour I, Salahi E, Pazouki M. Comparative of the removal of Pb 2+, Cd 2+ and Ni 2+ by nano crystallite hydroxyapatite from aqueous solutions: Adsorption isotherm study. Arabian J Chem. 2012;5:43946.
- Yang X, Lu X, Zhang Q, Zhang X, Gu Z, Chen J. BCP coatings on pure titanium plates by CD method. Mater Sci Eng. 2007;27:781-6.
- Rigo E, Boschi A, Yoshimoto M, Allegrini S, Konig B, Carbonari M. Evaluation in vitro and in vivo of biomimetic hydroxyapatite coated on titanium dental implants. Mater Sci Eng. 2004;24:647-51.
- Yang Y, Kim K-H, Ong JL. A review on calcium phosphate coatings produced using a sputtering processan alternative to plasma spraying. Biomaterials. 2005;26:327-37.
- Nimb L, Gotfredsen K, Steen Jensen J. Mechanical failure of hydroxyapatite-coated titanium and cobaltchromium-molybdenum alloy implants. An animal study. Acta orthopaedica Belgica. 1993;59:333-33.
- Geesink R, de Groot K, Klein C. Bonding of bone to apatite-coated implants. J Bone Joint Surg, Brit Vol. 1988;70:17-22.
- Zhang Q, Leng Y, Xin R. A comparative study of electrochemical deposition and biomimetic deposition of calcium phosphate on porous titanium. Biomaterials. 2005;26:2857-65.
- Forsgren J, Svahn F, Jarmar T, Engqvist H. Formation and adhesion of biomimetic hydroxyapatite deposited on titanium substrates. Acta Biomaterialia. 2007;3:9804.
- Bigi A, Boanini E, Bracci B, Facchini A, Panzavolta S, Segatti F, et al. Nanocrystalline hydroxyapatite coatings on titanium: a new fast biomimetic method. Biomaterials. 2005;26:4085-9.
- Jehn HA, Hofmann S, Rückborn VE, Münz WD.
- Morphology and properties of sputtered (Ti, Al) N layers on high speed steel substrates as a function of deposition temperature and sputtering atmosphere. J Vacuum Sci Tech A. 1986;4:2701-5.
- Ding S-J. Properties and immersion behavior of magnetron-sputtered multi-layered hydroxyapatite/titanium composite coatings. Biomaterials. 2003;24:4233-8.
- Vercaigne S, Wolke JG, Naert I, Jansen JA. A mechanical evaluation of TiO2-gritblasted and Ca-P magnetron sputter coated implants placed into the trabecular bone of the goat: part 1. Clin oral implants res. 2000;11:305-13.
- Nie X, Leyland A, Matthews A. Deposition of layered bioceramic hydroxyapatite/TiO 2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis. Surface Coatings Tech. 2000;125:407-14.
- Nie X, Leyland A, Matthews A, Jiang J, Meletis E. Effects of solution pH and electrical parameters on hydroxyapatite coatings deposited by a plasma-assisted electrophoresis technique. J biomedical mater res. 2001;57:612-8.
- Corni I, Ryan MP, Boccaccini AR. Electrophoretic deposition: from traditional ceramics to nanotechnology. J Eur Ceramic Soc. 2008;28:1353-67.
- Grandfield K, Zhitomirsky I. Electrophoretic deposition of composite hydroxyapatite-silica-chitosan coatings. Mater Charact. 2008;59:61-7.
- Hao J, Kuroda S, Ohya K, Bartakova S, Aoki H, Kasugai S. Enhanced osteoblast and osteoclast responses to a thin film sputtered hydroxyapatite coating. Journal of Materials Science: Mater Med. 2011;22:1489-99.
- Kuo M, Yen S. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater Sci Eng. 2002;20:153-60.
- Kim K-H, Ramaswamy N. Electrochemical surface modification of titanium in dentistry. Dent mater j. 2009;28:20-36.
- Faculty of Dentistry, Division of Oral Health and Society, McGill University, Montreal, Quebec, Canada.
- Faculty of Dentistry, Division of Prosthodontics, Ziauddin University, Karachi, Pakistan.
- Schulich School of Medicine & Dentistry, Western University, London, Ontario, Canada.
- Family Dental Clinic, Strathmore, Alberta, Canada.
- Faculty of Dentistry, University of Toronto, Ontario, Canada.
Corresponding author: “Dr. Haroon Rashid†< drh.rashid@hotmail.com >